吴恩达老师提出了一种反思翻译的大语言模型 (LLM) AI 翻译工作流程——具体工作流程如下: 1 提示一个 AI 将文本从 source_language 翻译到 target_language; 2 让 AI 反思翻译结果并提出建设性的改进建议; 3 使用这些建议来改进翻译。
早上一起床就分享分享了这个方案,也是码神我一直在用的翻译方案推荐给了社群群友,可能有的群友之前没接触过这些,可能不太熟悉,所有我就专门写一篇文章解释一下吧,群友的需求就是我的需求:D。
详细的解释 三步迭代流程(模型之间可以交叉反思,也可以从头到尾一个模型) 初始翻译 首先,使用 AI 模型进行初步翻译,相当于打草稿。这一步利用现有的模型,为后续优化奠定基础。 反思评判 接着,另一个 AI 对翻译结果进行思考和评判,找出可改进之处。这一步骤模拟了人类译者的自我审核过程,能够识别出初始翻译中的不足和潜在改进点。 优化翻译 最后,第三个 AI 根据反思结果对初始翻译进行优化,输出最终结果。这一步骤综合了前两步的输出,生成更加精准、流畅的翻译。
换成提示词步骤
初始翻译 源语言、目标语言、源文本这三个参数,生成提示词,传给 AI,让它给出第一版的翻译。
反思评判(用第一步生成的翻译结果) Write a list of specific, helpful and constructive suggestions for improving the translation. Each suggestion should address one specific part of the translation. Output only the suggestions and nothing else.
这里的提示词接收 5 个参数,源文本、初始翻译、源语言、目标语言以及限定词地区国家,这样 LLM 会对前面生成的翻译提出相当多的修改建议,为后续的提升翻译作准备。 3. 优化翻译
Output only the new translation and nothing else.
实操 原文 关于医疗器械的一些常识你了解吗?要是不了解的话,赶紧来看看这篇文章吧! 1.如何区分药品与含有药物成份的医疗器械? (1)对于产品中由药品起主要作用、医疗器械起辅助药品作用的(如预装了药品的注射器等),按药品管理。 (2)对于产品中由医疗器械起主要作用、药品起辅助作用的(如含药支架、带抗菌涂层的导管、含药避孕套、含药节育环等),按医疗器械管理。 (3)含抗菌、消炎药品的创口贴按药品管理。 (4)中药外用贴敷类产品作为传统的中药外用贴敷剂,按药品管理。 2.我国对医疗器械产品生产实行什么样的管理制度? 我国对医疗器械实行产品生产注册制度。 生产第一类医疗器械,由设区的市级人民政府药品监督管理部门审查批准,并发给产品生产注册证书。 生产第二类医疗器械,由省、自治区、直辖市人民政府药品监督管理部门审查批准,并发给产品生产注册证书。 生产第三类医疗器械,由国务院药品监督管理部门审查批准,并发给产品生产注册证书。 3.经营医疗器械产品需具备什么资格? 开办第一类医疗器械经营企业,应当向省、自治区、直辖市人民政府药品监督管理部门备案。开办第二类、第三类医疗器械经营企业,应当经省、自治区、直辖市人民政府药品监督管理部门审查批准,并发给《医疗器械经营企业许可证》。无《医疗器械经营企业许可证》的,工商行政管理部门不得发给营业执照。 经过几轮反思最终的结果(英文水平好的可以看下,结果) Are you familiar with some basic facts about medical devices? If not, take a moment to read this article! How can you differentiate between drugs and medical devices that contain drug components? (1) For products where the drug plays a major role and the medical device serves a supporting function (such as pre-filled drug syringes), they are regulated as drugs. (2) For products where the medical device plays a major role and the drug serves a supporting function (such as drug-eluting stents, antibacterial-coated catheters, drug-coated condoms, drug-releasing intrauterine devices, etc.), they are regulated as medical devices. (3) Adhesive wound dressings containing antibacterial and anti-inflammatory drugs are regulated as drugs. (4) Traditional Chinese medicine topical patches are regulated as drugs. What management system does China use for medical device production? China implements a product registration system for medical devices. The production of Class I medical devices is reviewed and approved by the drug regulatory authorities of municipal-level people’s governments with districts, and a product registration certificate is issued. The production of Class II medical devices is reviewed and approved by the drug regulatory authorities of provincial, autonomous regional, and municipal people’s governments, and a product registration certificate is issued. The production of Class III medical devices is reviewed and approved by the drug regulatory authorities of the State Council, and a product registration certificate is issued. What qualifications are required to sell medical devices? To establish a Class I medical device business, it must be filed with the drug regulatory authorities of provincial, autonomous regional, and municipal people’s governments. To establish a Class II or Class III medical device business, it must be reviewed and approved by the drug regulatory authorities of provincial, autonomous regional, and municipal people’s governments, and a “Medical Device Business License” is issued. Without the “Medical Device Business License,” the local Administration for Market Regulation shall not issue a business license.
在前文生成了初始翻译以及相应的反思后,将这二者输入给第三次 AI 翻译,这样我们就能获得一个比较高质量的翻译结果。免去了去找外援翻译,也能省下一大笔开支。(注意每一种模型可能效果不一样,建议多试试)
具体工作流的用户可以参考我之前发的教程。 https://mp.weixin.qq.com/s/ORmF7E5i76OWtLGlfWPkrQ
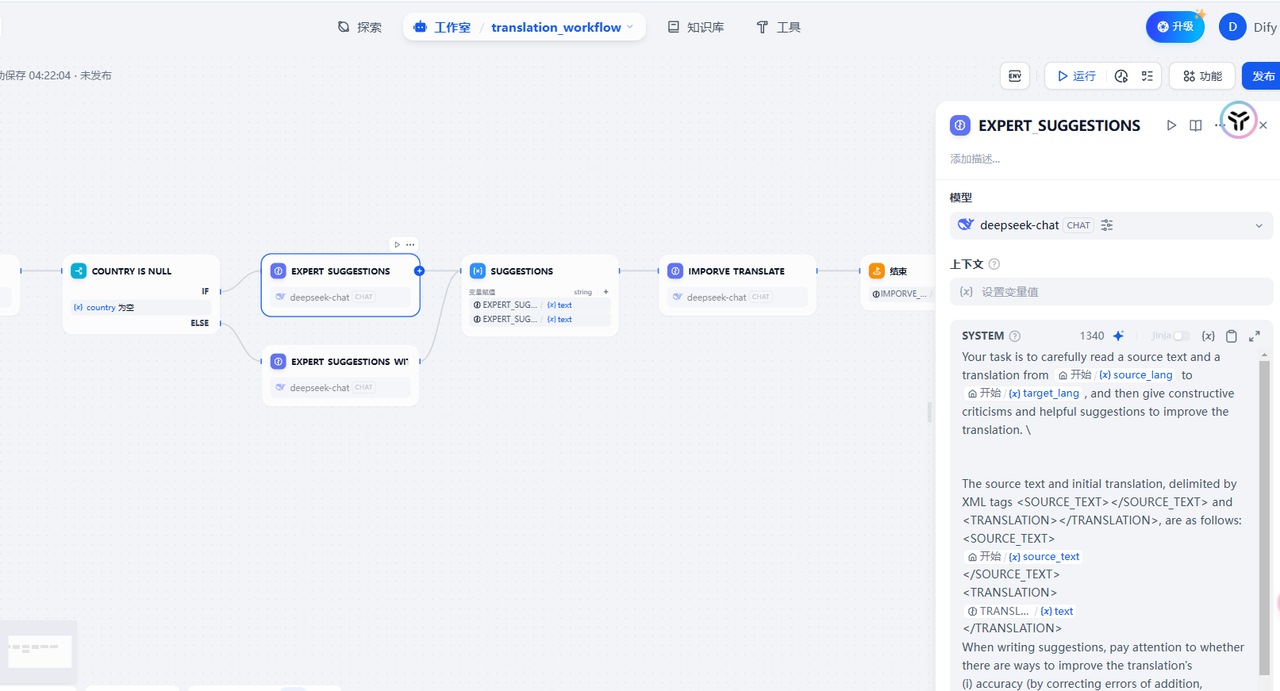
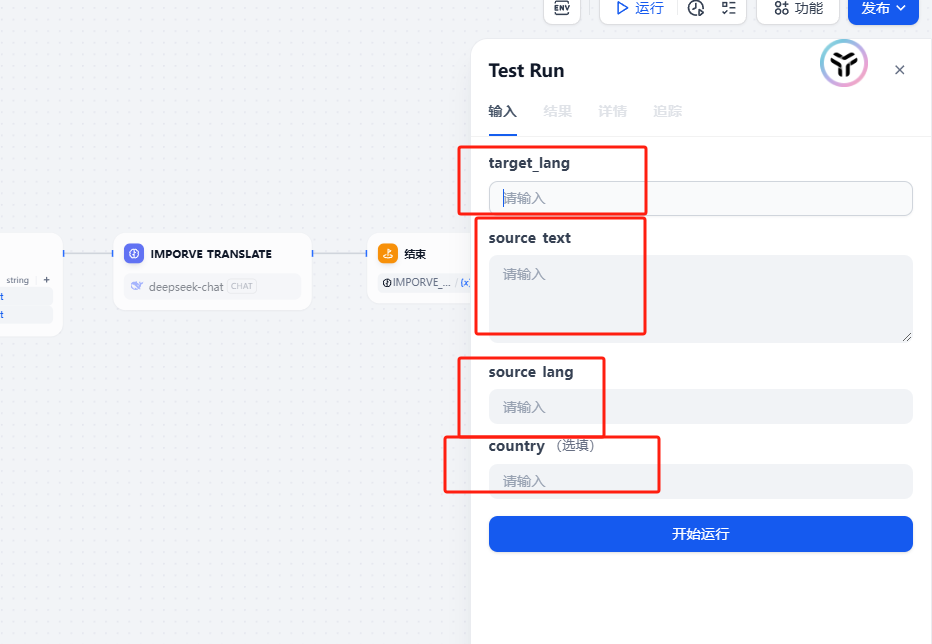 以上流程我用采用了工作流自动化生成,方便了很多,只需要输入参数即可,有需要的可以联系我获取工作流文件
以上流程我用采用了工作流自动化生成,方便了很多,只需要输入参数即可,有需要的可以联系我获取工作流文件